Biosimilars insights
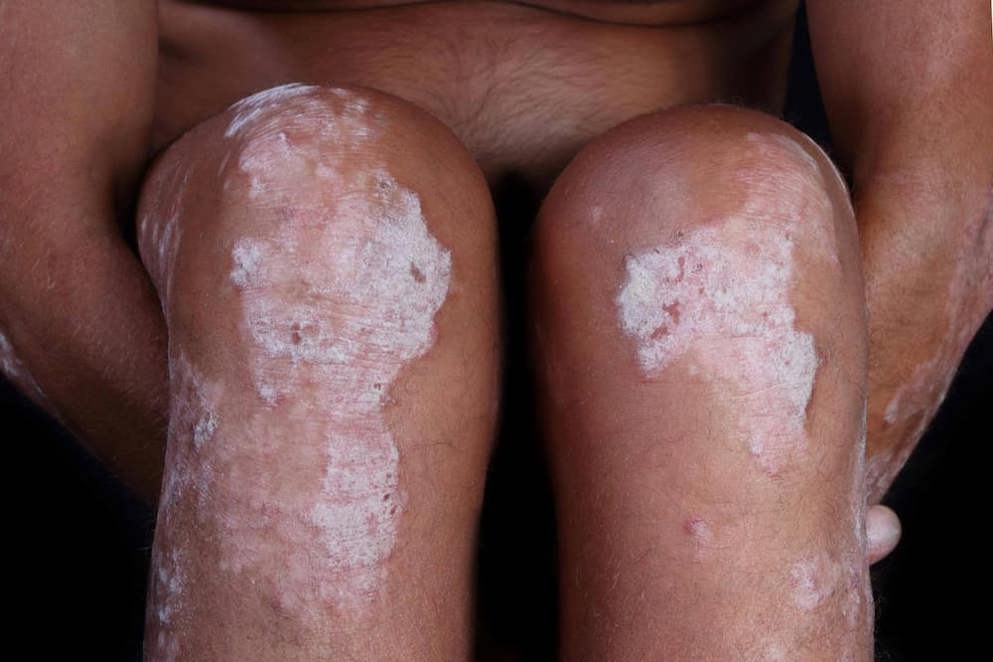
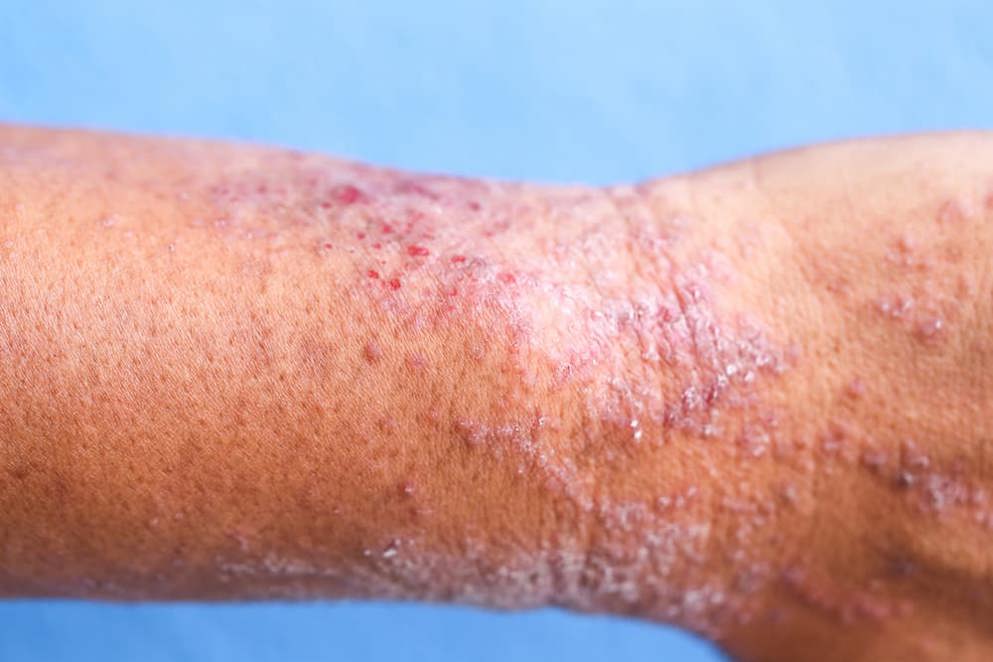
Biosimilars: an option to alleviate the heavy burden of immune-mediated inflammatory diseases
Developed by EPG Health for Medthority in collaboration with Biogen®. This content is intended for healthcare professionals only, and it has been funded and reviewed by Biogen® for scientific accuracy.
Clinical value beyond price alone: biosimilars improve patient access to treatment
Developed by EPG Health for Medthority in collaboration with Biogen®. This content is intended for healthcare professionals only, and it has been funded and reviewed by Biogen® for scientific accuracy.
Clinical value beyond price alone: anti-TNF biosimilars enable early treatment
Developed by EPG Health for Medthority in collaboration with Biogen®. This content is intended for healthcare professionals only, and it has been funded and reviewed by Biogen® for scientific accuracy.