News
FDA approves topical Wynzora Cream to treat plaque psoriasis.- MC2 Therapeutics
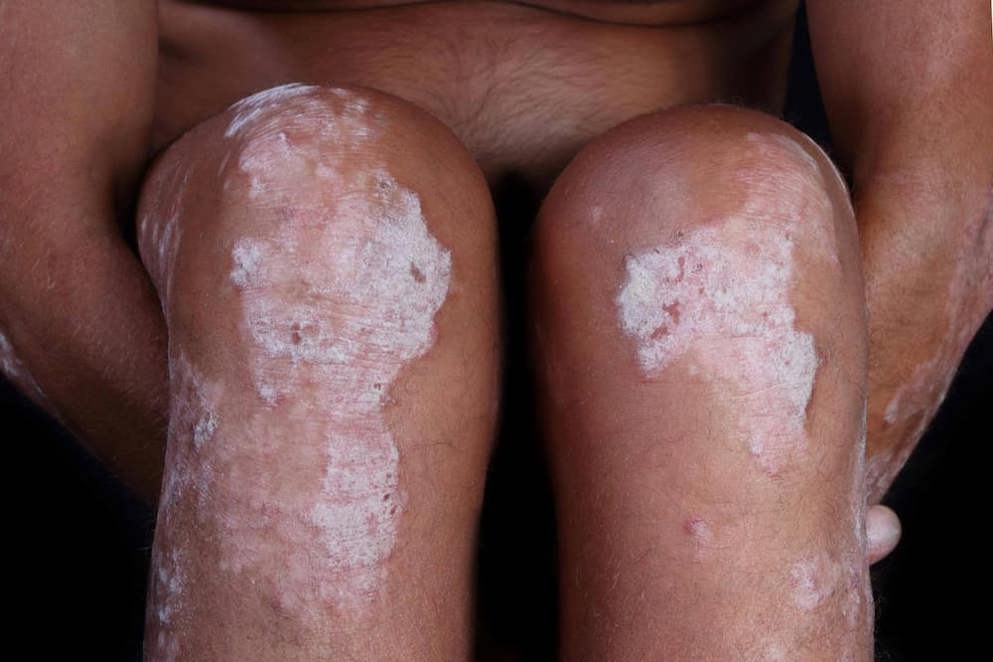
MC2 Therapeutics, announced that the FDA has approved Wynzora Cream (calcipotriene and betamethasone dipropionate, w/w 0.005%/0.064%) for once-daily topical treatment of plaque psoriasis in adults 18 years of age or older. The FDA approval is based on the results of the US Phase III clinical trial against active comparator Taclonex Topical Suspension (calcipotriene and betamethasone dipropionate, w/w 0.005%/0.064%).
A total of 794 patients were randomized in this trial and the primary efficacy endpoint was the proportion of patients with PGA treatment success at week 8 defined as at least a 2-grade improvement from baseline in PGA to “clear” or “almost clear”. The difference in PGA treatment success to the active comparator was 14.6% (95% CI; 7.6%, 21.6%) in favor of Wynzora Cream. Reduction of itch as defined by at least a 4-point improvement in the 11-point peak pruritus numeric rating scale (NRS) from baseline to week 4 was assessed among patients who had at least a peak pruritus NRS score of 4 at baseline. A higher proportion of patients achieved at least a 4-point improvement in the peak pruritus NRS score at week 4 in the Wynzora Cream group (60.3%) compared to vehicle (21.4%). Studies show that more than half of psoriasis patients are dissatisfied with their treatment and that a large proportion of patients are not treated at all.
With the US approval, the recent submission of its marketing authorization application of Wynzora Cream in EU, and its ongoing interactions with payers, physicians and patient organizations MC2 Therapeutics is well on track to launch Wynzora Cream in major territories. In addition, MC2 Therapeutics continues development of its pipeline of new topical therapies within major chronic inflammatory indications such as atopic dermatitis, uremic pruritus, lichen sclerosus and dry eye.
Condition: Psoriasis
Type: drug